ELENA BESSONOVA/iStock through Getty Images
Intro
Rhythm Pharmaceuticals ( NASDAQ: RYTM) is a biopharmaceutical business that concentrates on establishing and advertising treatments for unusual congenital diseases of weight problems. Its lead drug, Imcivree (setmelanotide), is a melanocortin 4 receptor agonist utilized to deal with weight problems due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), leptin receptor gene (LEPR) shortage, or Bardet-Biedl syndrome verified by hereditary screening.
The following post serves to information Rhythm’s potential customers and dangers in hereditary weight problems conditions.
Financials
Let’s very first evaluation Rhythm’s latest monetary report
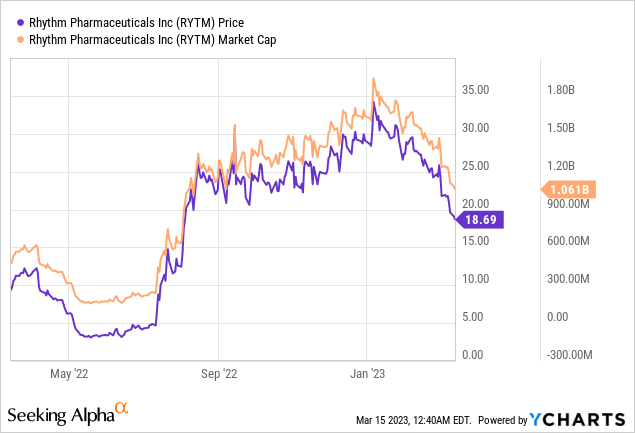
Rhythm Pharmaceuticals had $333.3 million in money and short-term financial investments since December 31, 2022. Item profits, web, from Imcivree was $16.9 million for the year ending December 31, 2022, a boost from $3.2 million in 2021. The business’s research study and advancement expenditures were $108.6 million, and selling, basic, and administrative expenditures were $92.0 million in 2022. For the year ending December 31, 2022, the bottom line was $181.1 million, compared to $69.6 million in 2021.
Comprehending Hereditary Reasons For Weight Problems and Their Management
Weight problems is a complicated condition brought on by a mix of hereditary, ecological, and behavioral aspects. One group of genes that have actually been linked in the advancement of weight problems is the POMC, PCSK1, and LEPR genes These genes play a vital function in the guideline of cravings and energy balance.
Anomalies in the POMC or PCSK1 genes can trigger a shortage in the production or processing of the hormonal agent melanocortin, which is accountable for indicating the brain to decrease food consumption and boost energy expense. Likewise, anomalies in the LEPR gene can hinder the body’s reaction to the hormonal agent leptin, which manages cravings and metabolic process.
Another congenital disease related to weight problems is Bardet-Biedl syndrome, which impacts numerous genes and is defined by weight problems, vision loss, and other signs.
Beyond setmelanotide, there are no particular treatments for hereditary reasons for weight problems. Nevertheless, the management of weight problems in people with POMC, PCSK1, or LEPR gene anomalies can consist of way of life adjustments such as a healthy diet plan and workout. In many cases, other medications or bariatric surgical treatment might be thought about, however their effectiveness in people with hereditary reasons for weight problems is not well developed.
For people with Bardet-Biedl syndrome, treatment includes the management of the different signs related to the condition, consisting of weight problems. This might include a multidisciplinary technique with a group of health care specialists, consisting of a nutritional expert, eye doctor, and endocrinologist.
Imcivree for Hereditary Weight Problems Conditions
Imcivree (setmelanotide) is a drug that works by triggering melanocortin 4 receptor (MC4R) in the brain, which is associated with managing cravings, satiety, and energy expense. It is thought that Imcivree can help in reducing food consumption and promote weight reduction in clients with weight problems brought on by unusual congenital diseases, such as POMC, PCSK1, or LEPR shortage, or Bardet-Biedl syndrome (BBS) related to inadequate MC4R path activation.
According to Rhythm,
Our public health quotes are around 4,600 to 7,500 for U.S. clients in preliminary FDA-approved indicators, consisting of weight problems due to biallelic POMC, PCSK1 or LEPR shortages, and BBS. Public health approximates for clients with hypothalamic weight problems is in between 5,000 and 10,000 in the United States, based upon our analysis of released literature and our public health approximates for the indicators being studied in our Stage 3 EMANATE trial recommend that around 53,000 U.S. clients with among these genetically driven weights problems have the prospective to react well to setmelanotide.
Research Study Outcomes: Imcivree’s Effectiveness in Clients with Hereditary Weight Problems
Clients with homozygous or assumed substance heterozygous pathogenic, most likely pathogenic variations, or VUS for either the POMC or PCSK1 genes ( Research Study 1) or the LEPR gene ( Research Study 2) were registered. Clients with double heterozygous variations in 2 various genes were not qualified for treatment with Imcivree. The clients went through a dosage titration duration for 2 to 12 weeks, followed by a 10-week open-label treatment duration with Imcivree. Clients who lost a minimum of 5 kgs or 5% of their body weight throughout this duration continued into a double-blind withdrawal duration of 8 weeks, consisting of 4 weeks of Imcivree followed by 4 weeks of placebo. After the withdrawal duration, clients resumed treatment with Imcivree at their restorative dosage for as much as 32 weeks.
In Research study 1, the effectiveness analysis was carried out on clients with POMC or PCSK1 gene shortages who finished a minimum of one year of treatment at the prespecified information cut-off. Out of these clients, 80% attained a weight reduction of ⥠10% after one year of Imcivree treatment. In Research study 2, the effectiveness analysis was carried out on clients with LEPR gene shortages who finished a minimum of one year of treatment at the prespecified information cut-off. Out of these clients, 46% attained a weight reduction of ⥠10% after one year of Imcivree treatment. Clients who had actually lost a minimum of 5 kgs throughout the open-label duration and had actually treatment withdrawn restored weight, however subsequent re-initiation of Imcivree led to weight reduction.
Imcivree seems an efficient treatment alternative for handling persistent weight concerns in clients with weight problems brought on by POMC, PCSK1, or LEPR shortage. Nevertheless, the little sample size and open-label style of the research study limitation the generalizability of the findings. Nevertheless, the outcomes suggest that Imcivree has the prospective to use advantages for clients with these particular hereditary reasons for weight problems.
My Analysis & & Suggestion
Imcivree’s efficiency in dealing with weight problems due to unusual congenital diseases has actually made it a feasible treatment alternative for clients with POMC, PCSK1, LEPR shortages, or Bardet-Biedl syndrome. Since November 2020, the drug was authorized in the United States for clients with weight problems due to POMC, PCSK1, or LEPR shortage verified by hereditary screening. In June 2022, it got United States Fda (FDA) approval for dealing with weight problems in clients with Bardet-Biedl syndrome. In addition, in September 2022, the European Commission approved approval for the drug’s usage in the European Union for dealing with weight problems due to congenital diseases.
Regardless of Imcivree’s effectiveness, it deserves keeping in mind that the drug’s usage is restricted to particular congenital diseases, which just impact a little number of clients. Rhythm likewise requires to commit significant resources to handle and validate hereditary tests that validate whether Imcivree appropriates for usage. For that reason, Rhythm’s profits stream is originated from a fairly little market size. Significantly, the business carried out an effective Stage 2 trial for setmelanotide treatment in clients with hypothalamic weight problems, where most clients attained the main endpoint of a BMI reduction higher than 5%. As an outcome, a Stage 3 trial was started in early 2023. The business is likewise performing Stage 3 trials in genetically specified illness associated to the MC4R path, a Stage 3 pediatrics trial, and a Stage 3 switch trial checking a weekly solution of setmelanotide.
Nevertheless, there are a couple of reasons that I beware about advising financial investment in Rhythm Pharmaceuticals and Imcivree. First of all, the dependability and generalizability of the drug’s effectiveness information might be doubtful due to prospective unblinding brought on by skin darkening in clients treated with Imcivree, in addition to the little sample size and treatment period of medical research studies. Second of all, the drug’s security profile is not completely clear, and there is a danger, in my viewpoint, of major negative results emerging while it is on the marketplace.
Imcivree label ( FDA)
One specific issue is the capacity for the drug to aggravate anxiety and self-destructive ideation in clients who are currently vulnerable to these conditions, such as those with hypothalamic weight problems Regardless of the exemption of clients with self-destructive ideation and DSM-III conditions that might hinder research study compliance, depression/depressed state of mind (26%) and self-destructive ideation (11%) were reported in both adult and pediatric clients throughout medical research studies. Keep in mind, clients in real-world settings will not be thoroughly chosen after satisfying many requirements.
My evaluation of Rhythm’s stock is to “Prevent” it, however provided the restricted alternatives of Strong Buy, Buy, Hold, Offer, or Strong Offer on Looking For Alpha, I would recommend “Offer” as the closest representation of my viewpoint.
Threats to Thesis
When it comes to the dangers to my bearish thesis, there are a couple of prospective aspects that might modify the outlook for Rhythm Pharmaceuticals and Imcivree. One possible danger is the growth of the drug’s authorized usages to more indicators beyond the existing restricted congenital diseases. If Imcivree shows to be reliable in dealing with other types of weight problems or consuming conditions, it might substantially increase the marketplace size and profits capacity for Rhythm.
In addition, the continuous medical trials for illness associated with the MC4R path and hypothalamic weight problems might yield favorable outcomes, resulting in more FDA approvals and profits development for the business.
Another prospective aspect to think about is the possibility of competitors emerging in the market. While Imcivree is presently the only drug authorized for the particular congenital diseases it targets, other business might establish comparable treatments in the future, possibly cutting into Rhythm’s market share.
It is likewise worth keeping in mind that the security issues surrounding Imcivree might show to be less considerable than at first believed, and the drug might have a much better security profile than existing information recommends.
In General, while there are dangers to the bearish thesis on Rhythm and Imcivree, the issues relating to effectiveness information dependability, security, and restricted market size are considerable adequate to necessitate care. As such, my suggestion stays to “Offer” Rhythm’s stock.